Failure Analysis of Medical Device
Dentalhitec© is a leading company offering effective, innovative products that make life easier for dental care practitioners, while improving surgery success rate and patient comfort during anaesthesia phase. Dentalhitec develops, manufactures and commercializes the only device in the world allowing dental practitioners to perform all kind local dental anaesthesia injections, including intraosseous.
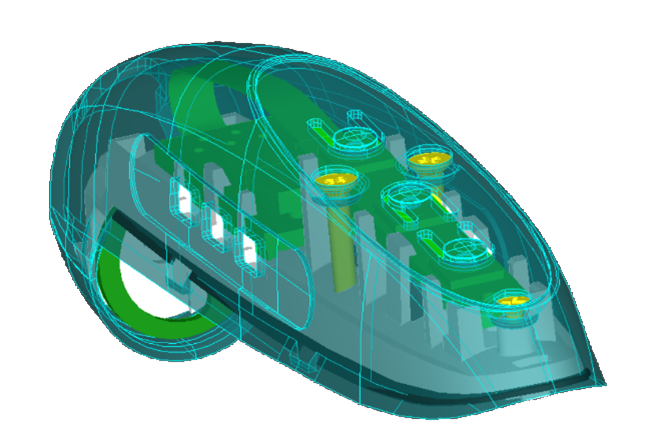


Dentalhitec was experiencing a concerning trend – random fractures occurring in a key component of one of their innovative dental anaesthesia devices Quicksleeper5®. These fractures compromised device integrity, potentially hindering practitioners in their mission of improving dental care.
Our team, with its extensive experience in Material and Structural Engineering, closely proposed to deploy a holistic life-cycle investigation for the device scrutinized components. Material analysis, design review, finite element analysis and manufacturing process review are areas where Oxybel and Dentalhitec closely collaborated to identify any potential contributing factors.
The implemented solutions effectively addressed the random fractures. Dentalhitec successfully launched the improved device, ensuring patient safety and device reliability. For Dentalhitec, this collaboration resulted in unimpeded innovation, savings due to the cost-effective solution and a stronger position on the market as a reliable provider in the dental technology market.
